The plasma for medicines programme
In February 2021, the Government lifted a decades old ban on using plasma from UK donors for immunoglobulin medicines, following a review by the independent experts of the Commission on Human Medicines. In June 2023, a similar ban on using UK plasma for albumin medicine was also lifted.
 The ban was introduced in 1998 in response to concerns over the spread of a human variant of BSE, known as 'mad cow's disease', called Creutzfeldt Jakob Disease. The UK had to rely on imports of these medicines.
The ban was introduced in 1998 in response to concerns over the spread of a human variant of BSE, known as 'mad cow's disease', called Creutzfeldt Jakob Disease. The UK had to rely on imports of these medicines.
Due to a large rise in global demand for immunoglobulins and albumin, there has been an ongoing pressure on supply in the UK and around the world for plasma derived medicines.
In April 2025 NHS patients began to receive plasma medicines made from the plasma collected from donors across England, for the first time in over 25 years.
Until now, we have been solely reliant on imports mostly from the US. There is a global shortage of plasma medicines, so it is really important we build up the capability to produce them ourselves
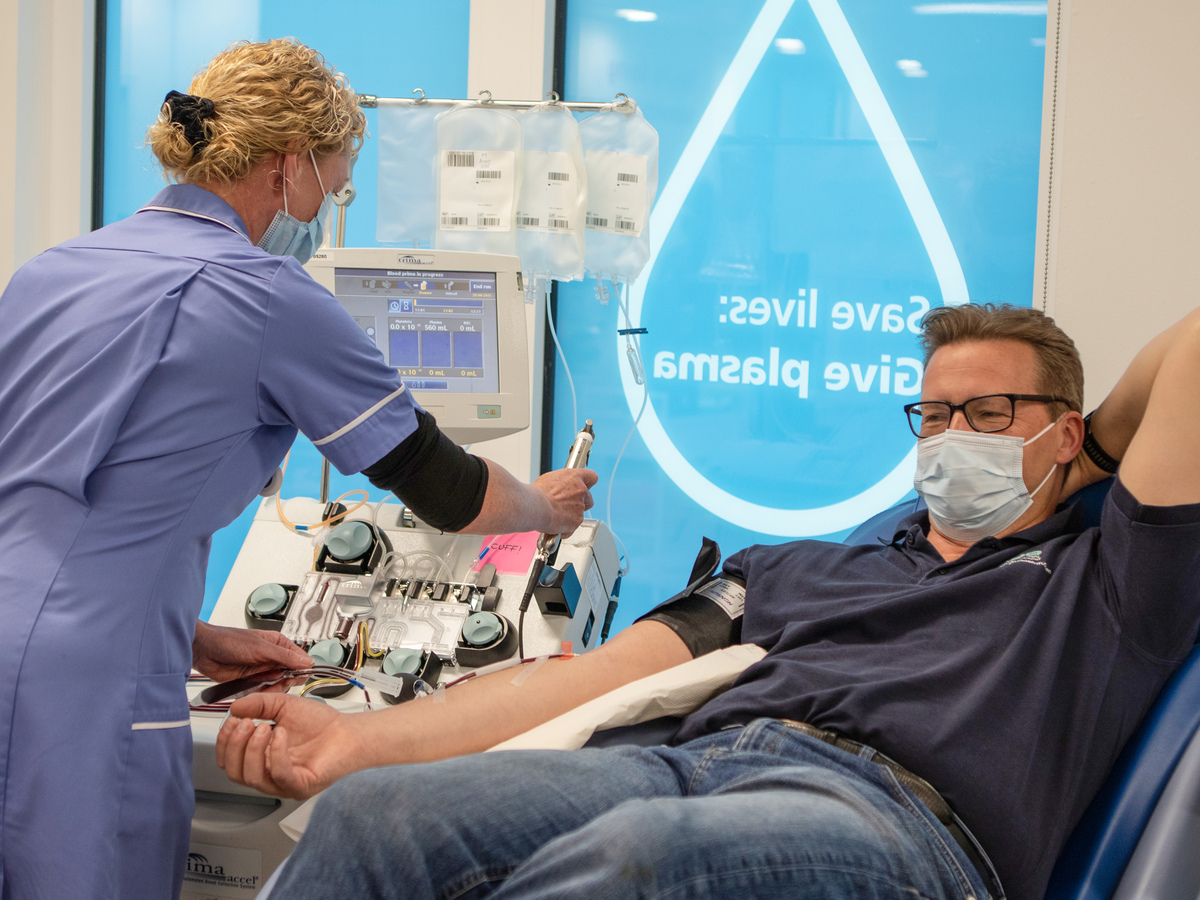
These lifesaving medicines can only be made from human blood and can only be made thanks to our amazing blood and plasma donors.
We have 3 plasma donor centres in Reading, Twickenham and Birmingham. We also recover plasma from blood donations across the country.
Octapharma were appointed by the Department of Health in 2023 as the fractionator to turn our UK plasma into medicines. The Department of Health and Social Care, NHS Blood and Transplant (NHSBT), and NHS England, are now working together on an ambition to create a long-term domestic supply of plasma in England
Does anyone sell plasma?
In some other countries, plasma derived medicinal products are made from plasma donated by donors who are paid. These medicines can be imported to the UK.
It is illegal to pay blood, plasma and platelet donors in the UK.
Does NHS Blood and Transplant sell plasma for profit?
No, NHS Blood and Transplant does not sell plasma for profit.
NHS organisations charge each other for the cost of services, on a cost recovery basis. For example, an NHS commissioning group might give an NHS hospital a fixed sum of money for a hip operation.
In the same way, NHS Blood and Transplant receives money for each unit of red cells, platelets and plasma that it supplies to hospitals. This money covers NHS Blood and Transplant’s costs.
Is NHS plasma donation privatised?
NHS plasma donation is not privatised. NHS Blood and Transplant is an NHS organisation. It is a Special Health Authority, reporting into the Department of Health and Social Care.
Supporting documents and useful links


