New NHS centre for making products for revolutionary cell and gene therapies
NHS Blood and Transplant (NHSBT) is today [Friday, March 10] opening a state-of-the-art facility which will contribute greatly to the UK’s ability to develop and manufacture new gene and cell therapies.
It will manufacture products for the development of potentially curative therapies for currently incurable diseases, such as some forms of cancer, sickle cell disease, and cystic fibrosis. Some of these will be personalised therapies, aimed at treating just one person.
The new Clinical Biotechnology Centre (CBC), which has been built in Bristol with a near £10m Government grant, is designed to expand the UK’s ability to make the clinical grade products required for the research and development of new cell and gene therapies.
It will support early phase clinical trials and pre-clinical work, providing a route to eventual commercial scale production.
It will help give patients quick access to the latest treatments by increasing the number of UK patients with incurable diseases who are able to take part in clinical trials and also bring new treatments into the NHS faster.
What are cell and gene therapies?
Cell and gene therapy is a cutting-edge area of medical development. Therapies are based on the idea that living cells or genetic material can be used to cure a wide range of acquired and inherited diseases, by altering their DNA or using them as a vehicle to deliver treatments. Gene and cell therapy can be used to treat illnesses such as leukaemia, haemophilia, autoimmune disorders, cancer, HIV, melanoma, and cystic fibrosis.
- Gene therapy works by fixing a genetic problem at its source. Genetic material, usually in a carrier such as a modified and inactivated virus, is transferred to cells, and the faulty DNA is replaced, inactivated, or repaired – for example, gene therapy is being used by the NHS to treat Spinal Muscular Atrophy. (1) Another example of Libmeldy, used to treat metachromatic leukodystrophy. (2)
- In cell therapy, the patient receives cells which then act with a therapeutic benefit. These cells are often genetically modified– for example, CAR-T therapy, where immune cells are modified to recognise and attack cancer cells. (3) Stem cell transplants or blood transfusions are examples of long-established cell therapies where the cells are unmodified.
Such advances in biotherapies offer new hope for patients for whom all other treatment options have been exhausted. CAR T-cell therapies have now been used to treat hundreds of patients.
How will the CBC help?
The CBC has been built at NHSBT’s base in Filton, in North Bristol, and replaces a smaller, ageing unit in nearby Langford. Larger commercial sites do exist, which are cost effective for making products for proven treatments. However, researchers need access to flexible sites where
they can cost-effectively make smaller amounts for treatments still being researched and clinically tested – that’s what the CBC will specialise in.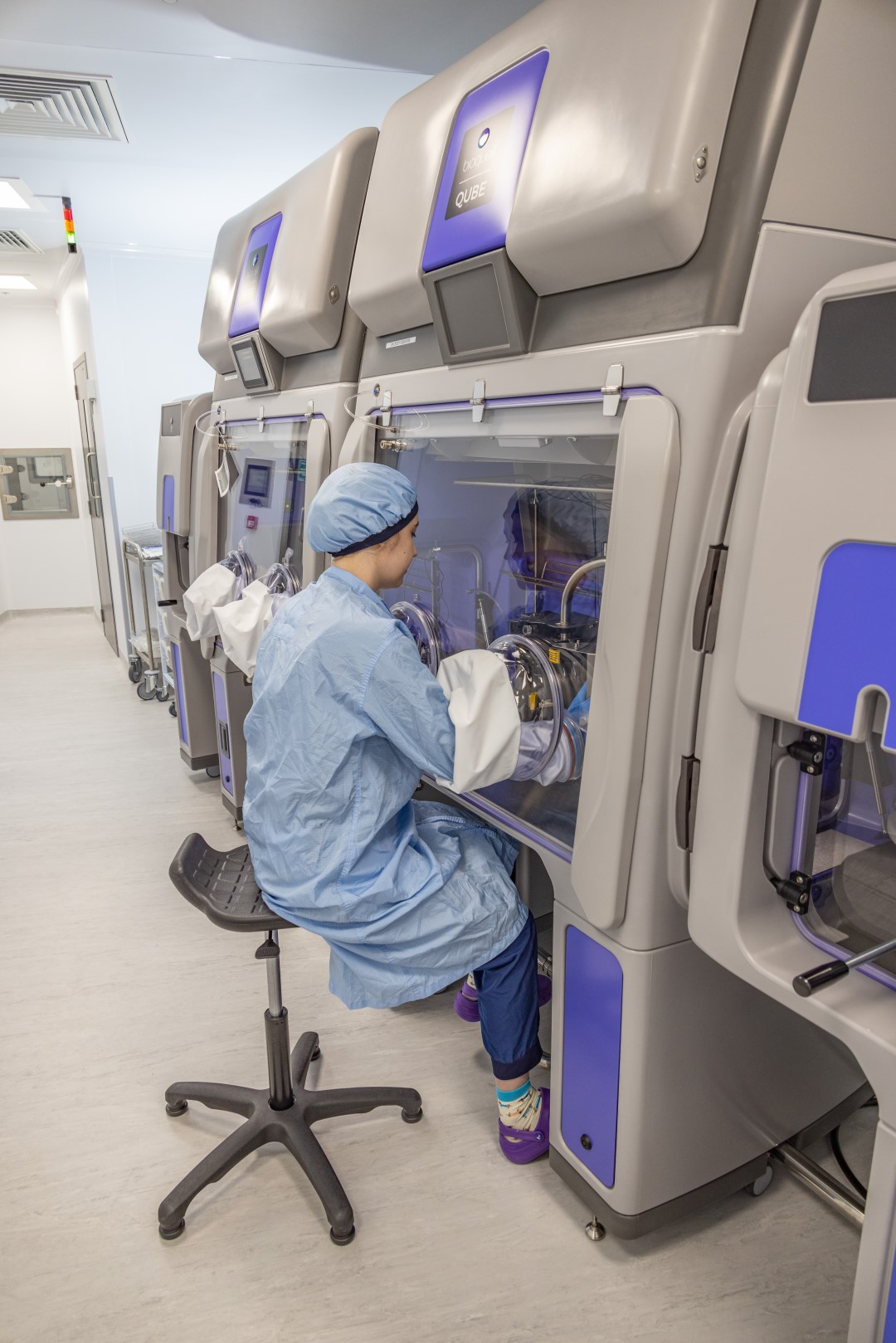
The UK currently has limited capacity to make the DNA plasmids and viral vectors used in the manufacture of gene therapies and genetically modified cell therapies. In particular, there is insufficient manufacturing at the scale required by organisations wishing to undertake early phase clinical trials of these biotherapies. The shortage of UK manufacturing capacity means long delays for developers of gene therapies while they wait for production slots.
Researchers often need to seek the services of overseas manufacturers, which inevitably delays clinical trials and patients’ access to much needed innovative therapies, and often increases costs. The new CBC will change that by expanding the UK’s ability to make its own plasmids and viral vectors. (4)
The CBC expansion was part funded by a Government grant of £9.43m. Expanding and improving the CBC’s unique offering is in line with the Government’s Life Sciences Industrial Strategy, which is to grow the UK’s manufacturing capacity for DNA-based therapeutics.
Nitya's story
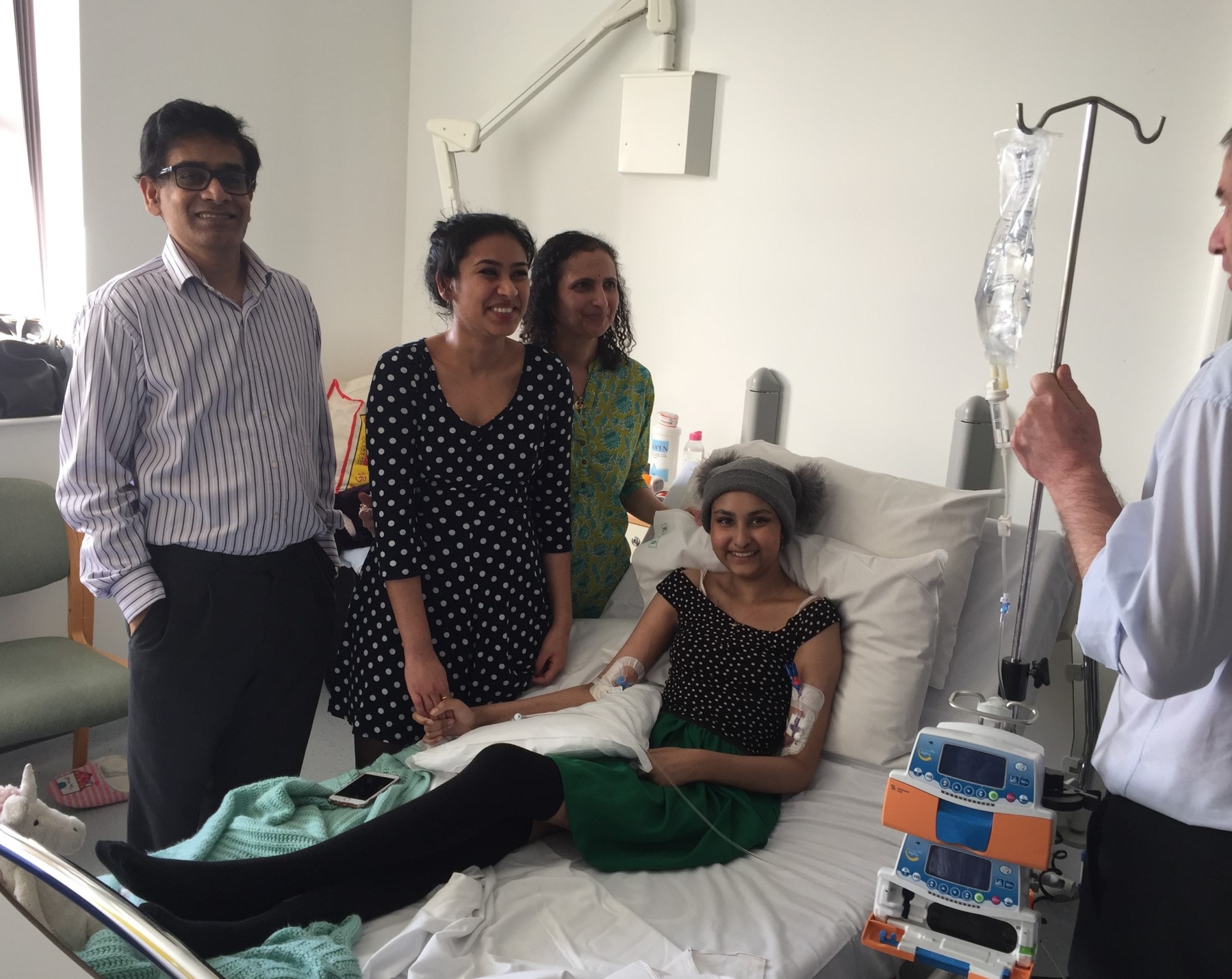 Nitya Raghava, 22, from Gloucester, had lifesaving CAR-T cell therapy for acute lymphoblastic leukaemia and will speak at the opening.
Nitya Raghava, 22, from Gloucester, had lifesaving CAR-T cell therapy for acute lymphoblastic leukaemia and will speak at the opening.
She was diagnosed through blood tests in 2016 after experiencing more than a month of fevers and headaches.
Nitya had treatment including chemotherapy and a stem cell transplant but after a relapse CAR-T was "pretty much a last resort."
Nitya went on to become the first person to receive CAR-T therapy at the Bristol Haematology and Oncology Centre in February 2019.
Nitya has now been free of the disease for four years. She is studying Spanish and Dutch at University College London.
She said: "I felt really excited to be able to receive CAR-T cells given they were new to the NHS."
"CAR-T was absolutely lifesaving for me. Without it, I don’t think I would be here."
"I think it’s just so exciting to see other new cell and gene therapies being developed at the CBC that can help other people too."
"I now feel great, I’m at university and I am living my life as normal. four years on from receiving CAR-T cells, because I’m in complete molecular remission with no evidence of disease now."
"I feel lucky that I got it when I did, and I hope more people also now get the chance to have new treatments. I hope the new CBC can help other new treatments to reach patients faster."
Press release notes
- Referenced from NHS England news website: NHS England strikes deal on life-saving gene-therapy drug that can help babies with rare genetic disease move and walk
- Referenced from NHS England news website: First baby receives life-saving gene therapy on NHS
- Information on CAR-T therapy available on NHS England website
- The CBC will make:
- Plasmids - Plasmids are small fragments of DNA, which can be engineered to introduce gene(s) of interest into cells. Our plasmids may be used for direct vaccination of patients (e.g. for HIV infection), cell therapies, or as the starting material for viral vectors.
- Viral Vectors – Viral Vectors are viruses that have been modified to deliver DNA into cells. They are commonly used in gene therapy for cell re-programming for a therapeutic effect.
- Recombinant proteins - Recombinant proteins are proteins with DNA combined from two or more sources. They are made by cells whose DNA has been altered. They have a wide range of uses including as medicines.