Lab grown red blood cells enable new malaria research
Dr Tim Satchwell is a Senior Research Associate working within the Toye Group as part of the NHSBT & University of Bristol NIHR Blood and Transplant Research Unit.
Often in science the biggest breakthroughs arise when efforts to achieve one research goal result in advances that can have major benefits or implications for another completely different area of study.
 My work for NHSBT is part of a broad research programme focused upon an ultimate goal to produce laboratory grown red blood cells to cater for patients with rare blood requirements that are difficult or impossible to meet through donations alone. More specifically, I study how proteins that are presented at the surface of the red blood cell and those that give the red blood cell its ability to squeeze through capillaries come together and interact as the cell develops. I am also trying to understand how newly made red blood cells (called reticulocytes) develop the ability to squeeze through the tiny capillaries in the circulation and using gene editing techniques such as CRISPR to explore and enhance the properties of lab grown red blood cells.
My work for NHSBT is part of a broad research programme focused upon an ultimate goal to produce laboratory grown red blood cells to cater for patients with rare blood requirements that are difficult or impossible to meet through donations alone. More specifically, I study how proteins that are presented at the surface of the red blood cell and those that give the red blood cell its ability to squeeze through capillaries come together and interact as the cell develops. I am also trying to understand how newly made red blood cells (called reticulocytes) develop the ability to squeeze through the tiny capillaries in the circulation and using gene editing techniques such as CRISPR to explore and enhance the properties of lab grown red blood cells.
One of the most important outcomes of the work that we have conducted as part of the Blood and Transplant Research Unit in Bristol in the past few years has been the development of an immortalised erythroblast cell line, the so-called BEL-A cell line. As groups across the world continue efforts to grow large amounts of red blood cells in the lab, this line provides a limitless supply of the precursor cells required to do this. Just as importantly, these cells provide a new research tool that allow us to study how these cells develop into red blood cells and have opened the door to the application of new gene editing techniques that enable us to manipulate their properties and characteristics.
In August I published a study that exploited these very capabilities, not for the generation of red blood cells for transfusion, but to establish a new model system that enables us to study how the parasites that are responsible for malaria are able to invade and develop inside red blood cells.
Proteins present on the surface of the red blood cell are known to play important roles during parasite attachment and invasion, and yet many unanswered questions remain regarding exactly how these proteins participate in or are exploited by the parasite during invasion. One reason for this is the fact that red blood cells have no DNA. This means that they cannot be directly genetically manipulated and has historically made answering these questions very difficult.
The BEL-A cell line enables red blood cells to be cultured in the laboratory starting from a source that allows unlimited numbers of immature ‘progenitor cells’ that do contain DNA to be grown. Using BEL-A cells, my collaborators and I were able to demonstrate that reticulocytes (young red blood cells) derived using this approach can support both invasion by and intracellular development of Plasmodium falciparum, the malaria parasite that is responsible for around 200 million new infections and half a million deaths per year worldwide.
Using CRISPR-based genetic manipulation, we were able to edit the genome of these cells in order to prevent expression a protein required for invasion and normally present on the surface of red blood cells called basigin. Red blood cells derived from this edited line and without surface basigin (a membrane and blood group protein) were completely resistant to invasion but the ability of the cells to support invasion could be restored by reintroduction of the basigin gene into the cells. This shows that these cells can be used to remove and replace different red blood cell proteins and assess how their absence or alteration affects the ability of the parasite to successfully invade.
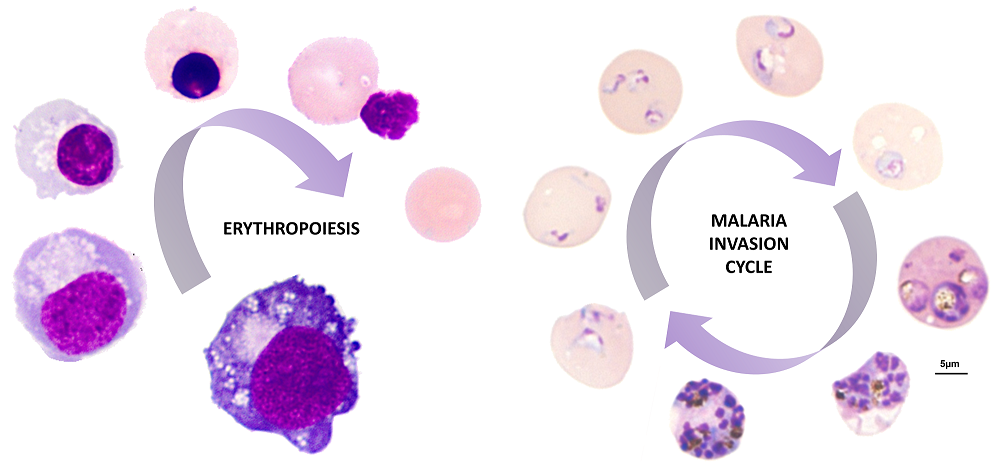
Figure 1: Images show the process of development of newly made red blood cell (reticulocyte) from a BEL-A erythroblast known as erythropoiesis and the invasion and intracellular development of the malaria parasite Plasmodium falciparum within BEL-A derived reticulocytes.
This work, which was recently published in the journal Nature Communications is the first demonstration of erythroblast cell line-derived reticulocytes supporting malaria parasite invasion and complete intracellular development. It represents a breakthrough that provides an important new tool for the research community to probe the mechanism of malaria parasite invasion and more specifically the role that the host red blood cell proteins play in this process.
Our efforts to more thoroughly understand the development and function of red blood cells and to generate them in the laboratory for therapeutic applications continue apace, harnessing an ever-increasingly sophisticated array of techniques to do so. However just as it is important that we learn from other areas and appreciate the insights into red blood cell function that can be gleaned from the study of disease, be it genetic e.g. sickle cells or in response to pathogen e.g. malaria; so it is important to recognise the impact our efforts can have for others and to exploit them as they arise. We look forward to more discoveries along the way!
