How bone marrow stem cells can support Acute Myeloid Leukemia to escape chemotherapy treatment
Dr Simón Méndez-Ferrer is an NHSBT Principal Investigator and a Reader in Transfusion Medicine at the University of Cambridge.
Haematopoietic stem cells (HSCs) are blood-forming cells that are found in the inner space of our bones. This inner space is called the bone marrow. The HSCs are supported by their surrounding tissue within the bone marrow, that maintains and regulates stem cells, called a ‘niche’ or bone marrow niche.
A century ago, haematologists realised that HSCs cannot survive or multiply on their own in the bone marrow, and that they require the support of neighbouring cells, the bone marrow stromal cells.
The bone marrow stromal cells are connective tissues cells, not blood forming cells and, are self-renewable.
There are different types of stromal cells, and one is called mesenchymal stem cells (MSCs). MSCs also reside in the bone marrow, and generate cartilage, bone and fat tissue. Our group found that MSCs are very important in supporting and regulating the production of HSCs.
Many cancers can originate in stem cells and unfortunately, they can re-emerge from those stem cells after therapy.
Bone marrow transplantation can be a curative treatment for some blood cancers, but up to half of all Acute Myeloid Leukaemia (AML) patients may relapse after a bone marrow transplant due to Leukaemic Stem Cells (LSC) that are resistant to chemotherapy. AML is a cancer of the blood and bone marrow.
Cancer cells were thought to arise solely by a damaged site in the cell’s DNA, called a DNA lesion. However, research over the past few years has revealed that what goes on outside the cancer cell is also important in cancer progression and the effectiveness of anti-cancer therapies.
For example, in experimental models of blood disorders, we found that the mutated HSCs damage their own bone marrow niche and cleverly avoid the bone marrow’s control enabling the mutated HSCs to drive the diseases development.
Therefore, the LSCs can then use the bone marrow niche for survival, multiplication (proliferation) and resistance to chemo-therapy. However, the underlying workings or pathways of this remain largely unknown, even though they could offer important clues as to how to develop new therapies for suppressing secondary tumour formation, to eradicate leukaemia.
As described in a manuscript published in 2020, we have studied the contribution of MSCs to AML disease development and chemoresistance in animal models (in vivo1).
This study shows that a group of MSCs that have the stem cell marker called ‘nestin’, directly support the survival and chemoresistance of LSCs. Importantly, the study shows that MSCs provide LSCs with the necessary tools to survive chemotherapy.
MSCs also increase energy production in the LSCs, which contributes to their high and fast growth in AML. Therefore, AML cells steal energy sources and defence mechanisms from MSCs to survive chemotherapy.
This study reveals specific pathways, that could be possible targets for secondary treatment therapies in AML, to overcome the protection generated by the bone marrow niche (see Figure 1).
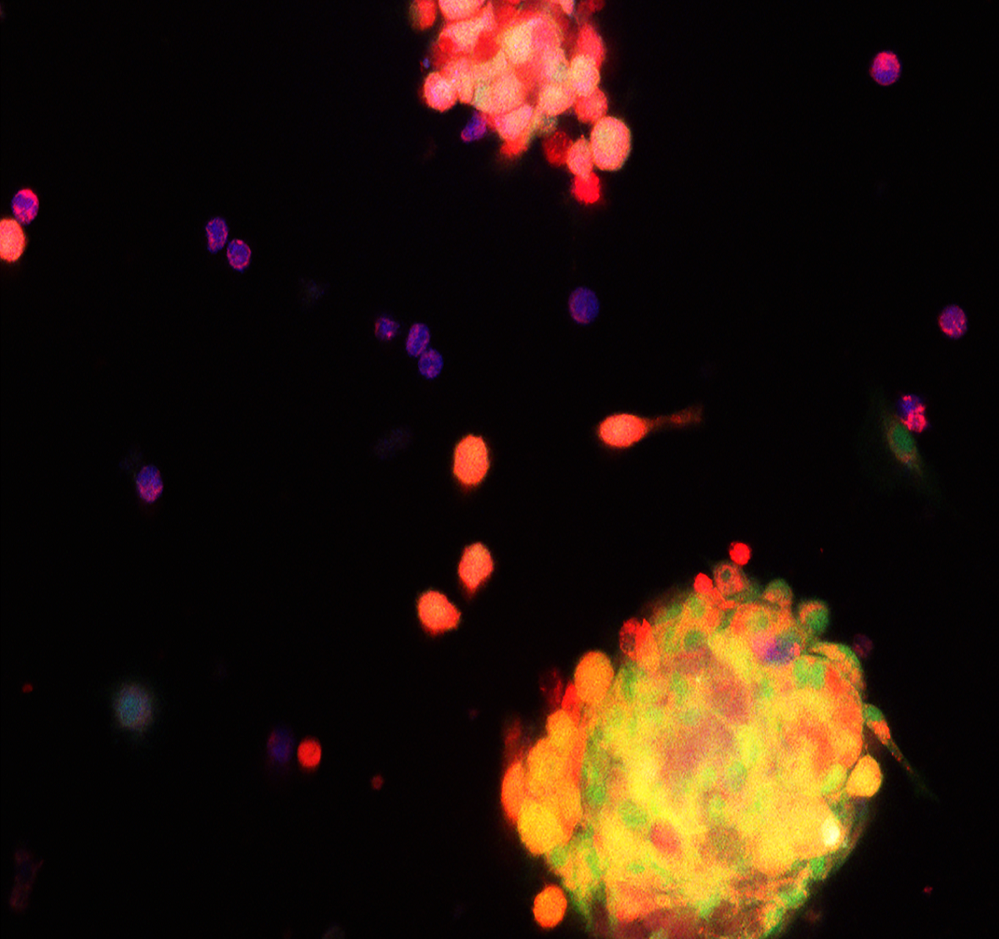
Figure 1. During chemo-therapy treatment, the energy-production centre inside the cell (mitochondria, labelled in red) are transferred from nestin+ MSCs (green) to AML cells, where they increase antioxidant molecules (blue) to survive chemotherapy.
