Tissue tracking
Our electronic audit trail tracks tissue from donor to recipient
Our tissue tracking process
It is a requirement of the EU Tissue and Cells Directive (EUTCD) that tissues are tracked from donor to recipient. We use the same computerised tracking system that is used to track blood donations (PULSE), which facilitates an electronic audit trail from donor to dispatch.
Hospital requirements
Hospitals are required to complete the audit trail from tissue receipt to patient or discard, and this requirement is included in the Service Level Agreement that all users must sign up to before tissue can be supplied. The EUTCD requires that the tracking method is documented in Standard Operating Procedures, hence these must be in place.
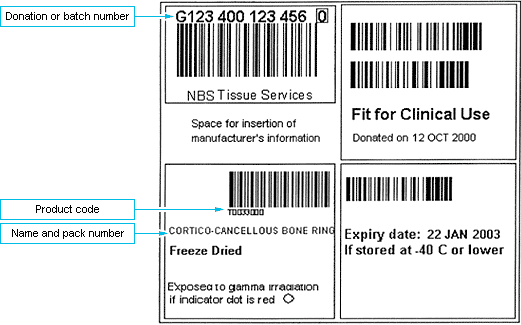
Tissue from us are labelled using the ISBT 128 labelling standard barcode format. This is described and defined in the Guidelines for the Blood Transfusion services in the UK, chapter 26.
We recommend, where possible, blood transfusion laboratories perform this task as they are familiar with and equipped for storage and tracking of clinical human derived products, and are audited to quality systems such as CPA. The labelling standard and layout is very similar to blood products, although blood products in the UK currently have a Codabar product and expiry date label whereas tissue products use full ISBT 128. The electronic tracking systems used by the blood transfusion department should be able to also track tissue products.
Please contact us on 0845 607 6820 if you require further information.
Tracking examples
Tracking can also be undertaken manually. An example of a simple tracking form is attached. The tissue is uniquely identified by 2 points on the label: Donation or batch number and the product code. As an alternative to writing the complete product code, the product name and pack number can be recorded.
Example of tissue tracking log which can also be used as an inventory.
You must also record a copy of the donation or batch number and product code in the patient's medical notes so that should an event occur possibly attributable to the tissue graft then the implicated unit can be quickly identified. This could be a photocopy of the product labels themselves to minimise transcription.
Order products
Call us on:
0845 607 6820
Fax:
0845 607 6819
New customer?
Find out what you need to know.
